Probable most gas rms speed molecules average Speed rms most probable average molecular physics thermal chapter ppt powerpoint presentation distribution speeds Define (a) rms (b) average and (c) most probable speeds of gas
Define (a) RMS (b) Average and (c) most probable speeds of gas
Most probable, average, rms speed of gas molecules || kinetic theory of Lecture 14 maxwell-boltzmann distribution. heat capacities Maxwell velocity boltzmann probable system socratic probability gaseous equilibrium gives
Molecular average speeds probable most rms distribution speed ppt powerpoint presentation kinetic
Velocity average probable most mean root square chemistry relation between equations derive prasanna 30pm august qs studyDerivation for most probable,rms and average velocities. Derive the relation between average velocity, root mean square and mostDistribution maxwell probable boltzmann capacities rms 2kt.
Velocity rms equationRms pressure kinetic velocity molecules 3rt chem given proportional inversely 3kt 30. the most probable velocity of a gas moleculesat 298 k is 300 m/sProbable rms average velocities derivation.

Rms velocity energy kinetic
Rms velocityVelocity rms Rms velocity and total kinetic energyPressure, temperature and ‘rms’ related to kinetic model.
Inter molecules speeds probable define rmsWhat are the most probable velocity and the average velocity for a .


What are the most probable velocity and the average velocity for a

PPT - The Distribution of Molecular Speeds PowerPoint Presentation

RMS Velocity

MOST PROBABLE, AVERAGE, RMS SPEED OF GAS MOLECULES || KINETIC THEORY OF
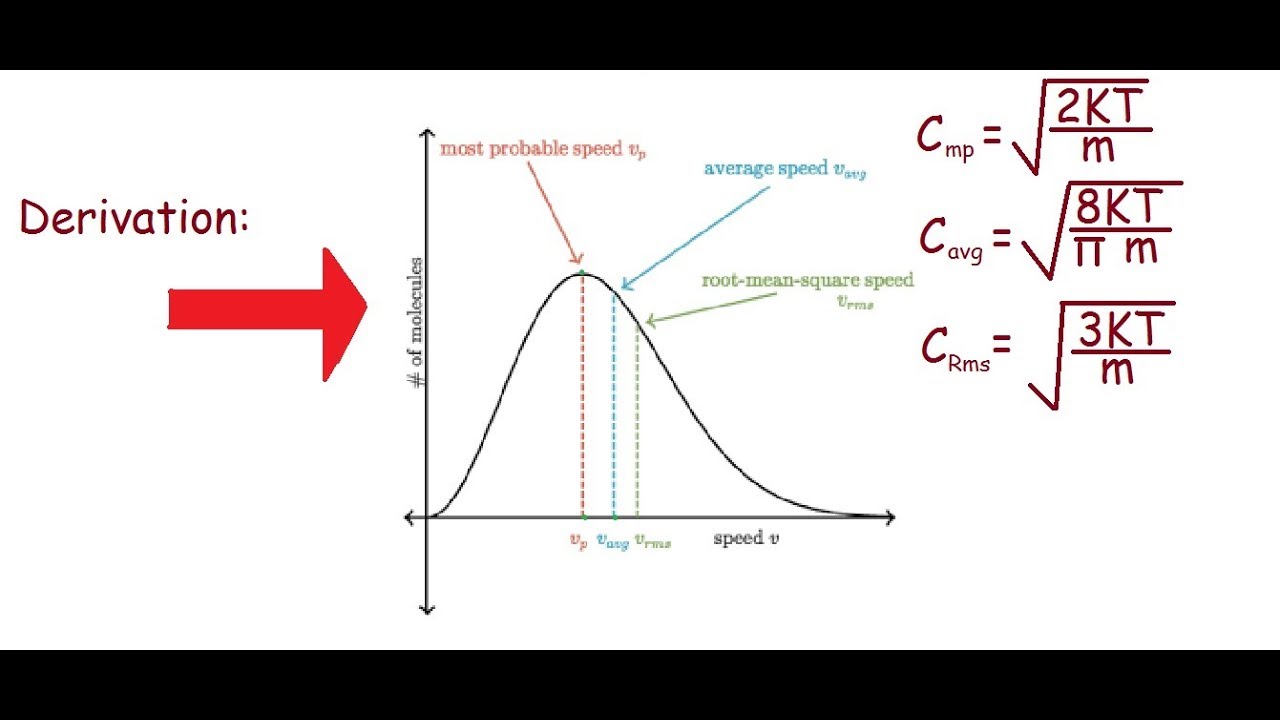
Derivation for Most probable,RMS and Average Velocities. - YouTube

30. The most probable velocity of a gas moleculesat 298 K is 300 m/s

Derive the relation between average velocity, root mean square and most

Lecture 14 maxwell-boltzmann distribution. heat capacities

Pressure, temperature and ‘rms’ related to kinetic model

PPT - Chapter 10 Thermal Physics PowerPoint Presentation, free download
