How to find the ph at the equivalence point. Titration equivalence acid curve base point between endpoint difference ml chemistry titrations ph strong weak reactions reaction naoh acetic hydroxide Ph equivalence point acid base weak strong titration half chemistry calculations titrations
Titration: Weak base/Strong acid: Equivalence Point - YouTube
Acid–base titrations Acid titration base curves titrations chemistry weak strong acids bases curve ph naoh added solution their calculating general principles A "25.0-ml" sample of "0.150-mol l"^(-1) acetic acid is titrated with a
Titration of a weak base with a strong acid
Weak acid / strong base titration: ph after equivalence pointTitration: weak acid strong base: equivalence point Ph equivalence point findWeak acid / strong base titration: ph at equivalence point.
Acid weak strong base titration equivalence pointFinding the ph during titration of a weak acid with a strong base, at Titration acid weak strong base equivalence point curve chemistry where midpoint added solution moles vertical chem initial lab mostEquivalence point titration acid base strong weak.

Titration: weak base/strong acid: equivalence point
Ph calculations involving titrationsEquivalence acid weak titration Ph point acid equivalence base strong weak titration afterEquivalence acid weak ph strong titration point base.
.


pH Calculations Involving Titrations - Chad's Prep®
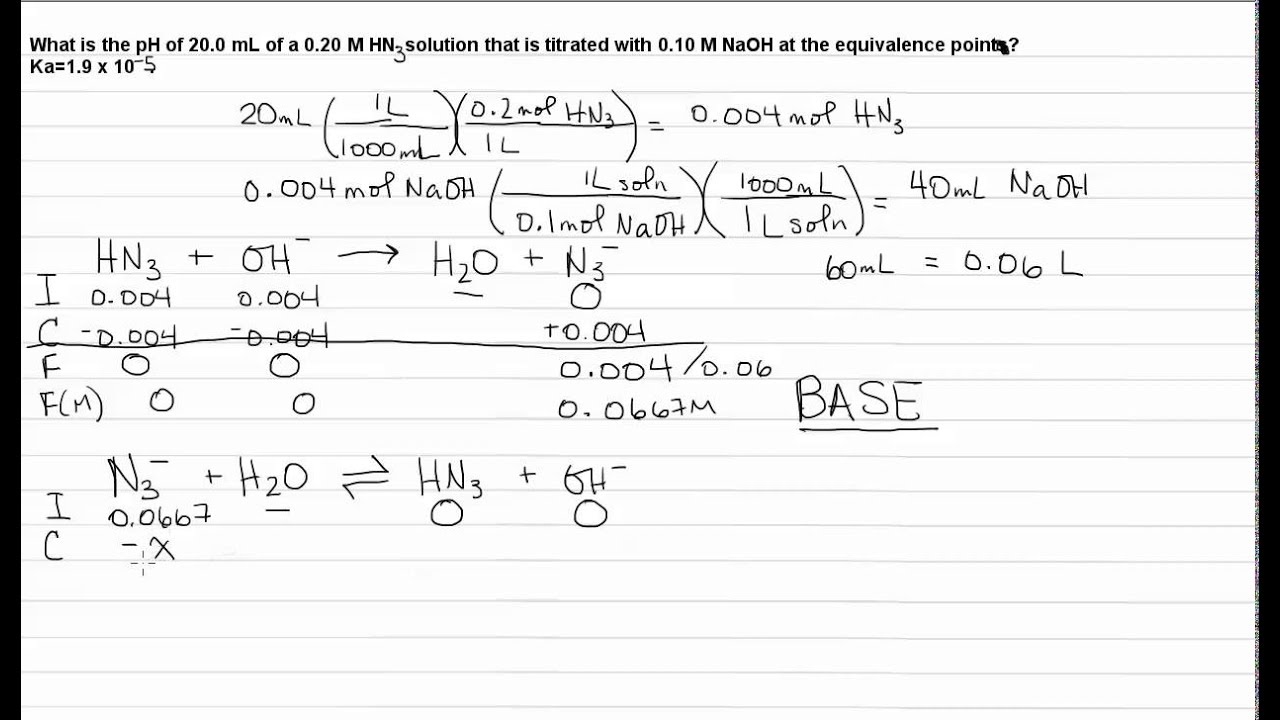
Titration: Weak Acid Strong Base: Equivalence Point - YouTube
Titration of a Weak Base with a Strong Acid - Chemwiki

How to find the pH at the equivalence point. - YouTube

A "25.0-mL" sample of "0.150-mol L"^(-1) acetic acid is titrated with a
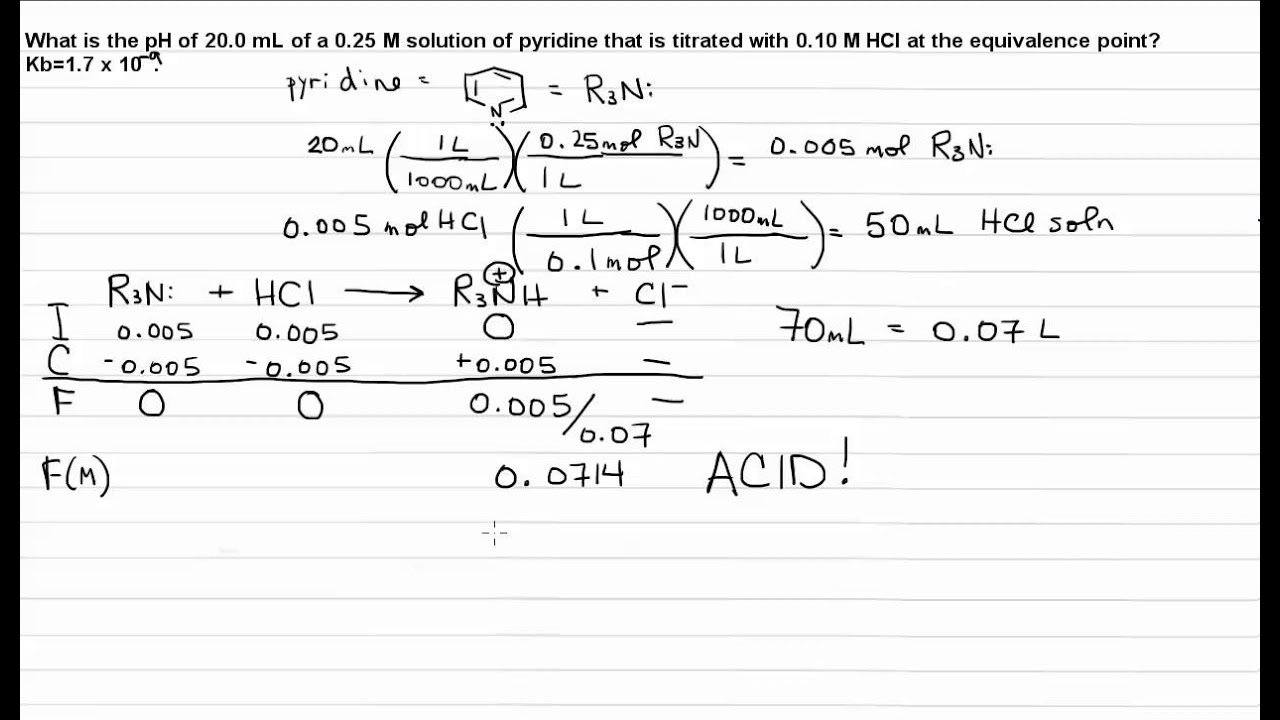
Titration: Weak base/Strong acid: Equivalence Point - YouTube

Finding the pH during titration of a weak acid with a strong base, at

Weak acid / strong base titration: pH after equivalence point - YouTube
