In the formation of n2+ from toppr.com N2 orbital toppr electron Lewis structure nitrogen n2 electrons valence octet atoms
What is the number of valence electrons in nitrogen? | Socratic
Unpaired electrons tardigrade n2 electron How many valence electrons does xenon (xe) have? [valency of xe] Electrons n2 molecule pair nitrogen pairs three electron atoms
The nitrogen atoms in a molecule of n2 share a total of (1) one pair of
Nitrogen covalent electrons valence molecule n2 bonding carbon sharing bonds socratic compounds thus octetA nitrogen molecule (n2) has one triple bond. how many electrons do the 2.2 quantum physicsN2 lewis structure ,valence electrons,formal charge,polar or non polar.
Electrons n2 nitrogen bond many triple molecule brainly atoms has do bonds covalent shared selectWhat is the number of valence electrons in nitrogen? Number of unpaired electrons in n2+ is/areXenon valence electrons xe valency periodic atomic.

Electrons quantum maximum principal orbitals n2 electron atom physics observed predicted shells technocrazed 2n2
.
.
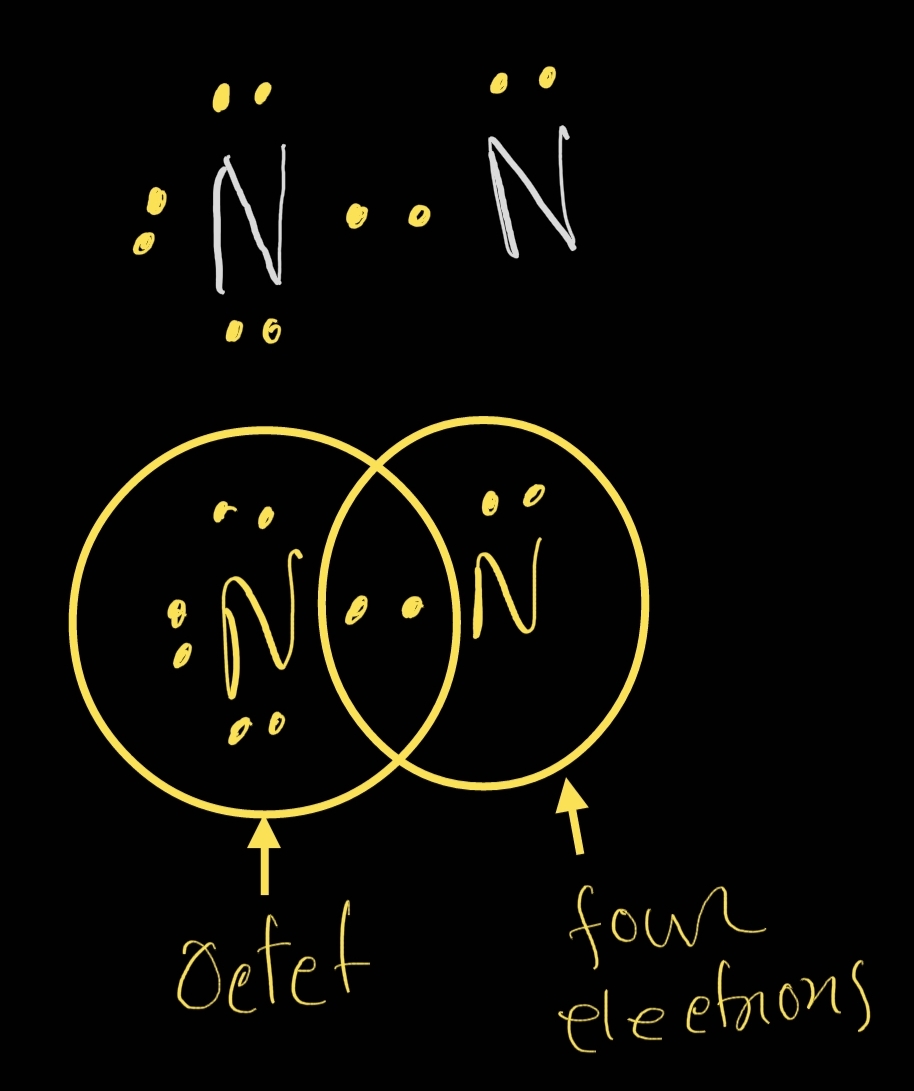

The nitrogen atoms in a molecule of N2 share a total of (1) one pair of

What is the number of valence electrons in nitrogen? | Socratic

In the formation of N2+ from toppr.com
![How Many Valence Electrons Does Xenon (Xe) Have? [Valency of Xe]](https://1.bp.blogspot.com/-4KmuZFEja_w/X5G7DtAyDTI/AAAAAAAACOE/-utcUQJQz6c_bsxlLTrLjhQlbjSfqsS6wCPcBGAYYCw/s1625/Screenshot%2B%252873%2529.png)
How Many Valence Electrons Does Xenon (Xe) Have? [Valency of Xe]

A nitrogen molecule (N2) has one triple bond. How many electrons do the

2.2 Quantum Physics
